Guest Blog by Dane B. Cook, PhD
On September 18, 2014, Dane B. Cook, Ph.D., Associate Professor of Kinesiology at the University of Wisconsin, Madison and a Solve ME/CFS Initiative 2011 funded investigator, presented a webinar on the system biology approach his team is taking to provide a clear picture as to what causes post-exertional malaise. You can access the full recording of the webinar HERE.
In this guest post for our blog, Dr. Cook reviews the material presented and tackles the many questions we received from webinar participants.
__________
Post-exertional malaise (PEM) has been called “the illness within the illness” (Anthony Komaroff, 2011). It is the defining and cardinal symptom of myalgic encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS), and yet the term used to describe it is a horrible one that does not do justice to the suffering that patients experience. Deciphering PEM is a critically important area of research for ME/CFS; you can’t begin to fix a problem like post-exertional malaise until you can understand its underlying cause. The goal of our research is to help our understanding of PEM so that we are able to mitigate its effects.
In the webinar I discussed both the clinical and laboratory characteristics of Post-Exertion Malaise (PEM) emphasizing that the patient experience and the laboratory study of PEM do not always coincide well with one another.
Clinical Perspective
There are several ‘descriptions’ or case definitions currently in circulation for ME/CFS. The international consensus criteria (Carruthers et al., 2011) offers a detailed clinical description of PEM and has potential utility for physicians. This case definition refers to PEM as Post-exertional neuroimmune exhaustion (PENE) and notes that it is a required symptom for the disease. It describes PEM or PENE as:
- Marked, rapid physical and ⁄ or cognitive fatigability in response to exertion, which may be minimal such as activities of daily living or simple mental tasks, can be debilitating and cause a relapse.
- Post-exertional symptom exacerbation: e.g. acute flu-like symptoms, pain and worsening of other symptoms.
- Post-exertional exhaustion may occur immediately after activity or be delayed by hours or days.
- Recovery period is prolonged, usually taking 24 h or longer. A relapse can last days, weeks or longer.
- Low threshold of physical and mental fatigability (lack of stamina) results in a substantial reduction in pre-illness activity level.
The current clinical description of PEM makes note of the large variability in time-course, severity and symptom make-up of this phenomenon among patients. This highlights the very real need for more research on these topics.
Research Perspective
Current clinical descriptions and case definition criteria of PEM are not currently quantified well-enough to be used in the laboratory setting. As a result, most studies have had to operationally define PEM. This has led to PEM being described and defined in a host of different ways including:
- Increased symptoms of pain & fatigue
- Reduced physical activity levels
- Abnormal exercise responses
- Changes in cognitive function
- Circadian rhythm/period changes
- Sleep disruption
- Impaired pain regulation (central nervous system sensitivity)
- Various biological markers (e.g. complement C4a, cytokines, natural killer cells, NF-кB, oxidative stress, gene expression, etc.)
Too many studies that have defined PEM based on biological outcomes have failed to measure symptoms. This is problematic because unless you demonstrate that biology is related to illness severity, you cannot attribute any changes in biology to the phenomenon of interest (in this case PEM).
The Solve ME/CFS Initiative funded a study led by my Master’s student Jacob Meyer. This study, published in 2013 in the journal Fatigue: Biomedicine, Health & Behavior, is an example of how we study PEM in the lab.
- This study examined gene expression for metabolite, immune and sensory receptors and symptoms pre and post exercise in ME/CFS patients and healthy controls who were matched on age and fitness. The study was an extension and replication of the work of Light and colleagues (2009; 2011) at the University of Utah.
- Results showed that maximal exercise resulted in sustained up-regulation of the glucocorticoid (NR3C1) and alpha 2A adrenergic receptors for up to 72 hours in ME/CFS patients compared to controls. Importantly, these changes in gene expression were significantly related to symptoms of pain, fatigue and confusion. These results demonstrate that ME/CFS patients have a different physiological response to exercise, one that appears to be consistent with exercise inducing an inflammatory state in ME/CFS patients and that clearly has negative consequences.
Our most recent study, “Post-exertion malaise in CFS/ME: Brain, inflammation and behavioral interactions”, also funded by SMCI, is a collaborative effort with Alan and Kathy Light at the University of Utah and Dr. Gordon Broderick at Nova Southeastern University in Florida. The project is a direct extension of our previous work (Meyer et al., 2013) and adds two critical components. The first is assessment of brain responses to cognitive tasks during a state of PEM. The second is the use of a “systems biology” approach to data analysis.
- The overall goal of this study is to determine the dynamic relationships between brain structure and function, gene expression for sensory, adrenergic and immune receptors and self-reported symptoms in CFS/ME using a model of PEM
- The key concept is that determining the interaction between several physiological systems (i.e. central and peripheral systems) will better predict PEM than determining the influence of any single system independently
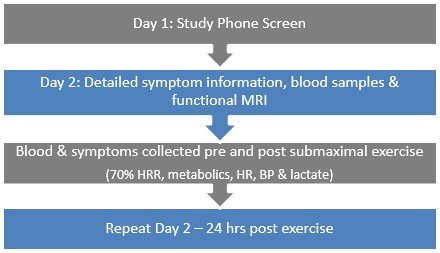
The study protocol:
- On Day one, we screen all participants for either ME/CFS or as healthy
- Day two consists of detailed symptom measurement and functional brain imaging of both fatiguing and non-fatiguing tasks. Blood is collected both prior to and following brain imaging.
- One week later participants return for exercise testing. A blood sample is taken and symptoms are measured. Participants exercise at 70% of peak heart rate for 30 minutes. Metabolic, heart rate and lactate responses to exercise are measured. Blood and symptoms are measured during the first 15 minutes post exercise.
- Participants return 24-hours later for their second functional brain imaging session (i.e. they repeat day two of testing)
Though this study is not yet completed, this is what our preliminary data is showing:
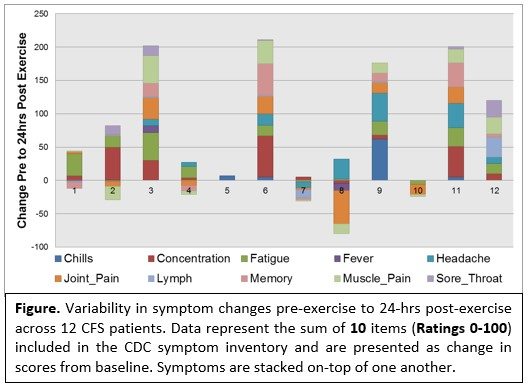
- Variability in symptoms from pre- to 24 hours post-exercise. These data demonstrate an incredible amount of variability in the magnitude, number and types of symptoms reported by patients. The variability is likely the result of a number of reasons such the severity of symptoms at baseline (i.e. ceiling effects), the individual time course of PEM (i.e. it may not have peaked for certain individuals) and how sick the patient was at study entry.
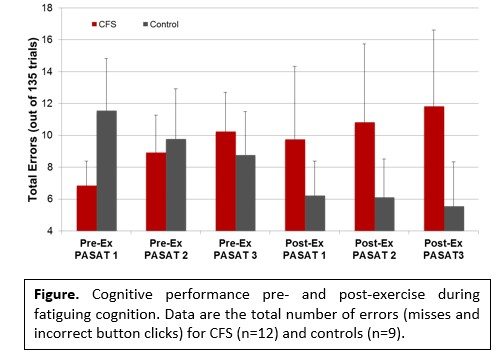
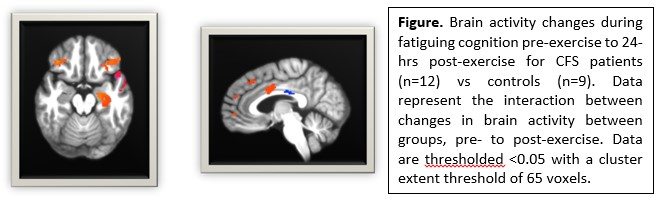
- Cognitive performance and brain responses for the fatiguing cognitive task. Patients tended to perform worse as they became more fatigued and performed more poorly post- compared to pre-exercise. The opposite was observed for controls. They tended to show improved performance during the first day and continued to improve 24 hours post-exercise.
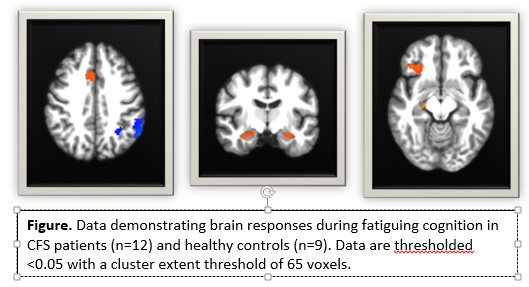
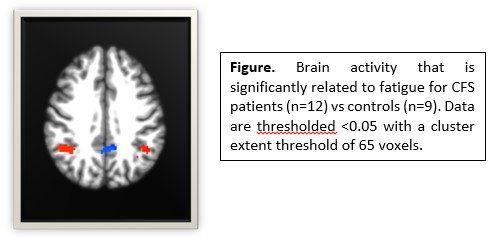
- Brain responses during the cognitive task reflected the cognitive performance results. ME/CFs patients showed greater brain activity than controls at baseline in brain regions involved in executive control (i.e. anterior cingulate and inferior frontal cortices) and memory (bilateral hippocampus).
Importantly, ME/CFS patients showed a different change in brain responses 24-hours post-exercise than controls and these changes in brain responses were significantly related to changes in symptoms. These data show that acute exercise has neural consequences for patients, suggesting that central nervous system outcomes are predictive of PEM.
My take home messages:
- Post-exertion malaise affects both the immune system and the brain.
- No single outcome or biochemical is likely to explain the complexity of PEM
- Determining physiological systems interaction will be critical towards understanding the mechanisms of PEM and perhaps how ME/CFS symptoms are maintained and/or exacerbated
- There is still a large gap in our knowledge of what PEM is in terms of time course, variability, severity and primary symptoms.
– # –
__________
This was our highest attended webinar of the year and there were far more questions submitted than we were able to cover during the webinar. Dr. Cook graciously took the time to answer a majority of the individual questions submitted and those responses are below.
Webinar Q & A
Q: Are there ME/CFS exercise studies with brain imaging before, during & after exertion?
A: To our knowledge only the study by Rayhan et al., (2013)in Gulf War Veterans with ME/CFS has looked at brain responses pre and post exertion.
Q: Why do you say “subjective” response & not “objective” to maximal exercise?
A: The subjective response I was referring to was the symptoms that the patients experience post exercise. We also focus on the objective responses to the exercise such as oxygen consumption.
Q: Are you taking in to consideration the age of your patients and the length of time ill when you look at your results?
A: We will look at this preliminarily, but with a sample size of 15 it may be tough to demonstrate a statistical effect. This is something we are certainly interested in and hope to be able to investigate in a larger study.
Q: Do you have a list of examples that you can offer about PEM to help physicians and others better understand that this is a disease within a disease?
A: Some personal examples:
- Need to stop grocery shopping mid-shop, stow shopping cart, lay-down in car for ½ hour and then return to finish shopping
- Completely unable to perform activities of daily living
- A debilitating crash following minimal exertion that can take weeks to recover from
A: Here is the 2011 case definition PEM list:
- International Consensus Criteria (Carruthers et al., 2011)
A. Post-exertional neuroimmune exhaustion (PENE): Compulsory
1. Marked, rapid physical and ⁄ or cognitive fatigability in response to exertion, which may be minimal such as activities of daily living or simple mental tasks, can be debilitating and cause a relapse.
2. Postexertional symptom exacerbation: e.g. acute flu-like symptoms, pain and worsening of other symptoms.
3. Postexertional exhaustion may occur immediately after activity or be delayed by hours or days.
4. Recovery period is prolonged, usually taking 24 h or longer. A relapse can last days, weeks or longer.
5. Low threshold of physical and mental fatigability (lack of stamina) results in a substantial reduction in pre-illness activity level.
Q: Have they found any muscle shut-down and/or myclonis with PEM?
A: Not to my knowledge
Q: Are you using functional MRI’s versus regular ones?
A: we are using both MRI (to look at brain structure) and fMRI (to look at brain function.
Q: Are you working with anyone in the Northwest (Portland)?
A: We are not currently working with Northwest US researchers.
Q: Can you tell us more about the effects seen with the HPA axis, particularly (eventually) effecting hormones: neuro, reproductive, thyroid?
A: It is too early to tell the cause or consequence of our glucocorticoid findings. Although the HPA-axis is an attractive hypothesis, the receptor response we are seeing could also be in response to inflammation. Some of the downstream consequences, if are results are confirmed, could mean that the neuroendocrine system is being affected in ME/CFS. This system regulates a host of hormones, the immune system, mood, storage and spending of energy. This system also interacts with numerous brain structures that interact with these systems.
Q: Can the different results of the symptoms be because there are not enough patients in the study?
A: This could certainly be a factor for average changes in symptoms for the group, however the individual variability we are seeing is not due to small samples. It’s showing us that PEM is highly variable and that we need large samples to study this variability in depth.
Q: Is IL2 refering to Interleukin 2?
A: Yes
Q: Is the PASAT the same version as for diagnosing MS?
A: I’m not sure if it is the same as used in MS research, but if not, it is very similar.
Q: Did this study include ME/CFS with fms??
A: We did not exclude for FM and yes some of our participants have both. We will look closely at this during our final analyses.
Q: Do repetitive episodes of PEM decrease a patients chance to recover from CFS/me?
A: I do not know the answer to this question, but it is a good one.
Q: Do these PEM abnormality show equally to M.E. AND Fibromyalgia patients?
A: This question is unknown (no data that I’m aware of) and it’s also a very good question.
Q: Do you think changes in brain scan images are a sign that there is brain damage or just a side effect of the illness?
A: The scans I presented do not tell us whether there is overt brain damage or not. They inform us if the structure of the brain is different in size and whether brain function differs during a particular task between patients and controls and in our case pre and post exercise.
Q: Is their lactic acid build up in muscles with exercise?
A: Yes, particularly for exercise that is of higher intensity.
Q: Does the up-regulation in in NR3C1 mean that cortisol level are extremely low or non-existent in the morning when cortisol levels are supposed to be the highest? And non-existent levels throughout the day known as adrenal fatigue?
A: The up-regulation we see could be in response to low levels initially or it could be in response to regular levels that don’t necessarily function optimally. Our data suggest that baseline levels of expression are not different between patients and controls, but this does not mean that the actual receptor numbers do not differ. That requires a different blood analysis.
Q: Were levels tested before exercise?
A: Yes, we tested levels at baseline. For our first study blood level for gene expression did not differ between patients and controls. I will add though that brain responses to cognitive tasks have differed at baseline.
Q: Have they studied Chronic Pain Patients in these same ways?
A: There are post-exertion malaise studies in chronic pain patients, but these tend to not have blood or brain outcomes. They tend to measure pain responses to pain stimuli.
Q: Is it possible to do research on patients that cannot function at a level to exercise for 30 minutes or do a 2 hour mental task? That IS the reality for so many of us. Even if you live in the area, just getting to the hospital for the research is a daunting outing.
A: At this point, I am not aware of studies that can accommodate the most severely affected patients. This is a sad and recognized limitation of this research. Once we learn more, perhaps we can investigate how little it takes to trigger PEM?
Q: Is it possible to do some of this testing in other places? What would be required? Equipment, training etc
A: Yes, we could design multi-site studies. It would require a significant funding level of course, but they could be done.
Q: Is there a difference in these tests if the person is new to the disease or someone who has had the disease a long time?
A: A great question and one we (and others) are interested in. At this point, we do not know.
Q: Is there any hope of a study to make sure that men show the same problems as women (i.e., that diagnosis and treatment should be the same for both)?
A: Mary Ann Fletcher just received an RO1 to look at sex differences in ME/CFS.
Q: Talk about the difference between post-exertional malaise and post-exertional relapse.
A: At this point, we don’t know the laboratory differences. Perhaps expert physicians would know the clinical differences?
Q: This is fabulous–the best webinar for me to share with family and friends to make them familiar with the condition. It would make it even better if it could be stated and printed in the PowerPoint that exercise tests are made to approximate the activities of daily living that cause PEM. I have had people respond with “just don’t exercise that hard.”
A: Thank you. I’m glad it was useful. My response to people who say “don’t exercise that hard” would be that research has shown that even light activity can result in a worsening of symptoms and to be honest we don’t know how much is too much.
Q: Were people in study suffering from ME/CFS only or have other medical conditions that may contribute to PEM? I am very healthy other than suffering from ME/CFS and PEM is one of the most severe symptoms, mentally and physiologically, I experience!
A: All patients had ME/CFS and no other medical explanation for their symptoms or intolerance for exercise.
Q: Does PEM typically have detrimental effects on the immune system?
A: The research to date suggests that PEM does affect the immune system. Some data suggest that these responses are detrimental, but there is much more we need to learn about the consequences of the changes that we are seeing.
Q: You mentioned brain inflammation, is there any way you have to measure that?
A: There are some neuroimaging techniques that get at brain inflammation. For example, MR Spectroscopy can be used to look at certain molecules in the brain that are indicative of brain cell health that might be related to brain inflammation.
Q: Do you find a delay in the response, at least in symptoms after the exercise?
A: There is research to suggest that symptom worsening can be delayed for many days. There is other research that shows symptoms get worse the day of exertion and stay bad for several days. We need more research to understand this variability better.